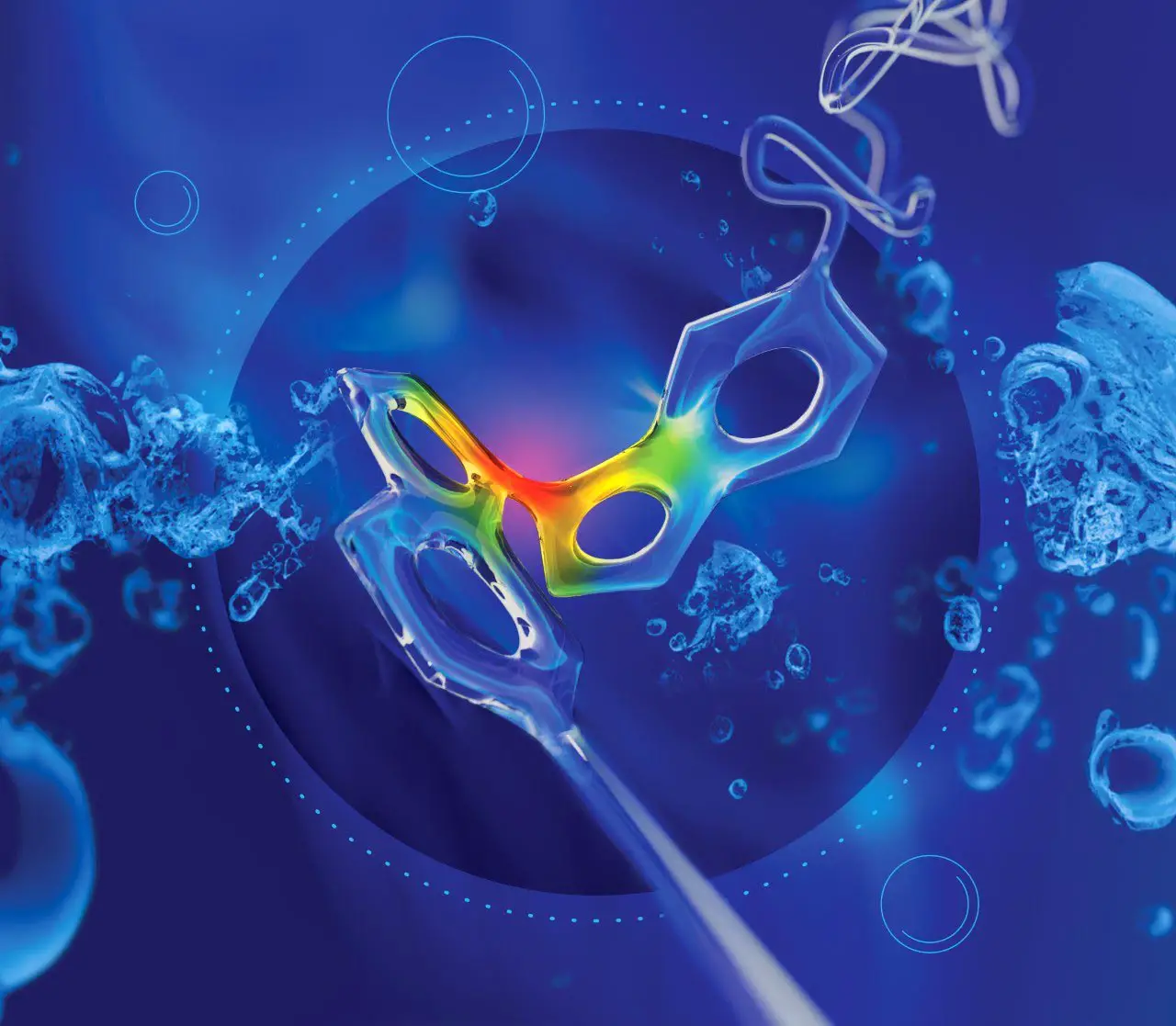
Artistic rendering of a polymer chain containing a molecular force probe (central structure) being distorted by the flow field around an imploding cavitation bubble (central circle). Credit: Professor Roman Boulatov, University of Liverpool
The University of Liverpool’s Chemistry Department has pioneered a method to better understand polymer chain reactions in changing solvent flows, offering valuable insights for both science and industries like oil recovery and photovoltaics.
New research by the University of Liverpool’s Chemistry Department represents an important breakthrough in the field of polymer science.
In a paper published recently in the journal Nature Chemistry and featuring on the front cover, Liverpool researchers use mechanochemistry to characterize how a polymer chain in solution responds to a sudden acceleration of the solvent flow around it.
This new approach allows a fundamental and technological question that has preoccupied polymer scientists for the past 50 years to be finally answered.
Historical Challenges and Implications
Since the 1980s researchers have been trying to understand the unique response of dissolved polymer chains to suddenly accelerating solvent flows. However, they have been constrained to highly simplified solvent flows that provided limited exploitable insights into the behavior of real-world systems.
The new discovery by Liverpool chemists Professor Roman Boulatov and Dr. Robert O’Neill has significant scientific implications for several areas of physical sciences as well as at a practical level for polymer-based rheological control used in many multi-million dollar industrial processes such as enhanced oil and gas recovery, long-distance piping and photovoltaics manufacturing.
Expert Insights
Professor Roman Boulatov said: “Our finding addresses a fundamental and technical question in polymer science and potentially upends our current understanding of chain behavior in cavitational solvent flows.”
Co-author of the paper, Dr Robert O’Neill added: “Our proof-of-the-approach demonstration reveals that our understanding of how polymer chains respond to sudden accelerations of solvent flows in cavitating solutions was too simplistic to support systematic design of new polymer structures and compositions for efficient and economical rheological control in such scenarios or for gaining fundamental molecular insights into flow-induced mechanochemistry.
“Our paper has important implications for our ability to study non-equilibrium polymer chain dynamics at the molecular length scales, and thus our capacity to answer fundamental questions of how energy flows between molecules and within them, and how it transforms from kinetic to potential to free energies.”
Future Endeavors
The research team plans to focus on expanding the scope and capabilities of their new method and exploiting it to map molecular-level physics that would allow accurate predictions of flow behavior for an arbitrary combination of polymer, solvent, and flow conditions.
Reference: “Experimental quantitation of molecular conditions responsible for flow-induced polymer mechanochemistry” by Robert T. O’Neill and Roman Boulatov, 10 July 2023, Nature Chemistry.
DOI: 10.1038/s41557-023-01266-2